Imagine a ‘Battery’ that Charges 100 Times Faster
by Chris Clarke – Apr 16, 2013
This week, another UCLA team reports it may have found a way to address a persistent problem with supercapacitors: limitations on their effective size.
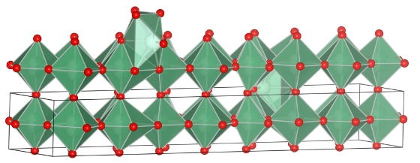
Researchers at UCLA’s Henry Samueli School of Engineering and Applied Science have found a way to use niobium oxide as a matrix to allow the fabrication of supercapacitors the size of batteries, but which could conceivably charge and deliver power hundreds of times as quickly as typical batteries can.
Batteries and supercapacitors differ in the way they store electrical power. Batteries store power in the form of chemical energy, and while that’s a very efficient way to store a considerable amount of power, it makes charging and power delivery relatively slow: electrons (or other charged particles, lithium ions being one common example) must work their way through a solid substance in both directions, which takes time.
Supercapacitors, on the other hand, essentially just “hang” those charged particles on a matrix without forcing them to migrate though a solid material, meaning that charging can take place as fast as the electrons can move. But the technology is limited by the fact that storage takes place only along the capacitor material’s surface area, which means that once the material gets larger than wafer-thin, its storage capacity per unit of weight drops.
Think of the two storage technologies as different parts of a movie theater, with electrically charged particles as moviegoers. The ranks of seats are like a battery: you can hold a whole lot of people there, but once the aisle seats fill up it can take some time to get people to the seats in the interior. Supercapacitors are more like the aisles: you can fill them and empty them really quickly, but they don’t hold nearly as many people.
The breakthrough by the team at the Samueli School of Engineering — led by led by professor of materials science and engineering Bruce Dunn — involved using a crystalline matrix of niobium oxide that’s essentially “porous” on a molecular scale: it’s got as much open space in its makeup as solid material, meaning it’s got a large expanse of internal surface area on which charged particles can be stored.
“With this work, we are blurring the lines between what is a battery and what is a supercapacitor,” said Veronica Augustyn, a graduate student in materials science told UCLA’s Bill Kisliuk. “The discovery takes the disadvantages of capacitors and the disadvantages of batteries and does away with them.” Augustyn is lead author of a paper describing the breakthrough, published April 14 in the journal Nature Materials.
The work suggests that electrodes 40 microns thick — a bit more than a thousandth of an inch — could be charged and discharged as efficiently as supercapacitors that are far thinner. And as Kisliuk points out, existing commercial batteries these days often use electrodes about that size.
Bruce Dunn closes Kisliuk’s post with the most optimistic obligatory cautionary disclaimer we here at ReWire have seen in quite some time:
Dunn emphasizes that although the electrodes are an important first step, “further engineering at the nanoscale and beyond will be necessary to achieve practical devices with high energy density that can charge in under a minute.”
by Chris Clarke
on February 21, 2013 2:51 PM
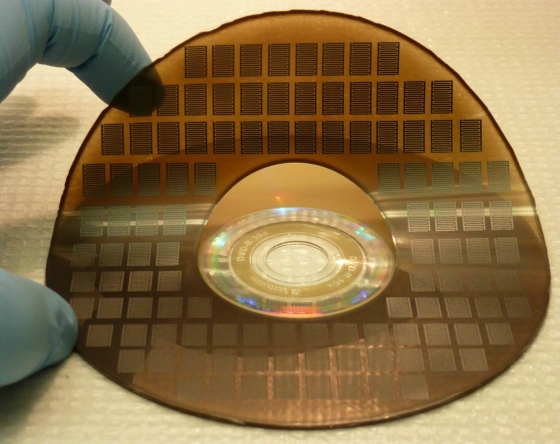
The recap: Graphene, a very simple carbon polymer, can be used as the basic component of a “supercapacitor” — an electrical power storage device that charges far more rapidly than chemical batteries. Unlike other supercapacitors, though, graphene’s structure also offers a high “energy density,” — it can hold a lot of electrons, meaning that it could conceivably rival or outperform batteries in the amount of charge it can hold. Kaner Lab researcher Maher El-Kady found a way to create sheets of graphene a single carbon atom thick by covering a plastic surface with graphite oxide solution and bombarding it with precisely controlled laser light.
English translation: He painted a DVD with a liquid carbon solution and stuck it into a standard-issue DVD burner.
The result: Absurdly cheap graphene sheets one atom thick, which held a surprising amount of charge without further modification.
That work was reported a year ago; we mentioned it due to the video virally making the rounds this week. Late Tuesday, UCLA announced that El-Kady and Kaner have a new article in press, in the upcoming issue of Nature Communications, describing a method by which El-Kady’s earlier, slightly homebrewed fabricating process shown in the video can be made more efficient, raising the possibility of mass production. As the authors say in their article abstract,
More than 100 micro-supercapacitors can be produced on a single disc in 30 min or less.
El-Kady and Kaner found a way to embed small electrodes within each graphene unit, and place the whole thing on a flexible substrate that allows the supercapacitor to be bent. The team is already claiming energy density comparable to existing thin-film lithium ion batteries.
In the video we shared Tuesday, Kaner says that this technology, if it pans out, offers possibilities like a smart phone getting a full day’s charge in a second or two, or an electric car reaching “full” in a minute. This week’s press release from UCLA offers other intriguing possibilities:
The new micro-supercapacitors are also highly bendable and twistable, making them potentially useful as energy-storage devices in flexible electronics like roll-up displays and TVs, e-paper, and even wearable electronics. The researchers showed the utility of their new laser-scribed graphene micro-supercapacitor in an all-solid form, which would enable any new device incorporating them to be more easily shaped and flexible. The micro-supercapacitors can also be fabricated directly on a chip using the same technique, making them highly useful for integration into micro-electromechanical systems (MEMS) or complementary metal-oxide-semiconductors (CMOS). As they can be directly integrated on-chip, these micro-supercapacitors may help to better extract energy from solar, mechanical and thermal sources and thus make more efficient self-powered systems. They could also be fabricated on the backside of solar cells in both portable devices and rooftop installations to store power generated during the day for use after sundown, helping to provide electricity around the clock when connection to the grid is not possible.
Kaner says that his lab is now looking for partners in industry that can help make these graphene supercapacitors on an industrial scale.
It’s tempting to be cynical about the possibility of a magic bullet energy storage solution; such a breakthrough could solve any number of problems from annoying dead smart phones to two-hour charge times for electric cars to an inefficient power distribution grid, and it’s easy to really want this kind of thing to be true. Plenty of seemingly promising technical innovations in the last few years haven’t lived up to their hopeful hype. There’s always the chance that further study will reveal a fatal flaw in graphene supercapacitor technology. But for the time being, ReWire officially has its hopes up, at least a little.





